MiCheck® Prostate
A risk score for clinically significant prostate cancer – MiCheck® Prostate: quick, non- invasive, highly sensitive and specific
Intended Purpose
MiCheck® Prostate is an algorithm that combines the testing results of three serum immunoassays with the patient’s clinical factors to calculate a MiCheck® Prostate Percentage Risk Score, providing an indication of the likelihood of the presence of clinically significant (i.e. aggressive) prostate cancer. The clinical factors that can be included in the algorithm are the patient’s age, result from digital rectal examination (DRE) or a multiparametric MRI (mpMRI)-derived prostate volume result.
MiCheck® Prostate is for male subjects being considered by physicians for a prostate biopsy. It is intended to supplement and not replace the significance of other clinical factors or test results which collectively assist the clinical judgement and experience of the urologist in the decision about whether or not to proceed to prostate biopsy. Prostate biopsy is required for the diagnosis of cancer.
Read More
Current prostate cancer testing regimes
PSA testing
PSA screening has significantly contributed to reducing prostate cancer mortality, with recent data demonstrating reductions in prostate cancer mortality of up to 35% following PSA screening [1]. However, an elevated PSA is not specific to prostate cancer, and it is frequently associated with benign conditions.
• Elevated PSA on its own is not a reliable indicator of prostate cancer, with only an estimated 18% of patients in the PSA 4-10ng/ml range having a positive biopsy[2].
• Elevated PSA can be caused by conditions other than prostate cancer, including prostatitis, enlargement of the prostate, bike riding and sexual activity,
• Elevated PSA cannot differentiate between patients without cancer, nor patients with non-clinically significant or clinically significant prostate cancer, and
• PSA results can vary according to the clinical analyser used, often leading to repeat testing.
mpMRI
MRI of the prostate is widely used in many countries, including Australia, following an elevated PSA. mpMRI enables visualisation of the prostate and grades the images using the Prostate Imaging Reporting and Data System (PI-RADS) classifications developed by the International Prostate MRI Working Group.
The PI-RADS system categorises prostate lesions based on the likelihood of cancer according to a five-point scale, defined as the following:
PI-RADS 1 – Clinically significant cancer is highly unlikely to be present.
PI-RADS 2 – Clinically significant cancer is unlikely to be present.
PI-RADS 3 – The presence of clinically significant cancer is equivocal.
PI-RADS 4 – Clinically significant cancer is likely to be present.
PI-RADS 5 – Clinically significant cancer is highly likely to be present.
Patients with PI-RADS scores of 4 and 5 will typically proceed to prostate biopsy, while those with scores of 1 and 2 will not. Biopsy decisions with patients with PI-RADS scores of 3 are particularly challenging, with clinically significant cancer rates of as low as 12%, compared to 60% for PI-RADS 4 and 83% PI-RADs 5[3]. Furthermore, there is no consensus on which PI-RADs 3 patients should proceed to biopsy. Despite current practice not recommending biopsy of patients with PI-RADS scores of 1 and 2, up to 18% of such patients can harbour clinically significant prostate cancer[4].
A significant limitation of mpMRI is that the interpretation of the PI-RADS scores vary considerably between operators, with one study demonstrating the significant cancer yield across 9 operators ranged from 3% to 27% for PI-RADS 3, from 23% to 65% for PI-RADS 4, and from 40% to 80% for PI-RADS 5 lesions[5]. Therefore, the PI-RADs 1-3 group of patients requires the development of new complementary technologies to assist urologists in making a clinical determination of which patients would benefit from proceeding to biopsy.
The USANZ issued a position statement on MRI for prostate cancer in 2016. Its recommendations were as follows:
Although primarily under the jurisdiction of the RANZCR, USANZ supports the following general principles:
- Prostate MRI should be ordered for specific indications as discussed below, and only by specialists managing prostate cancer (i.e., urologists, radiation oncologists and medical oncologists) – its use in primary care is not recommended.
- Prostate MRI should be undertaken in facilities with the appropriate equipment – usually, a 3 Tesla magnet (and suitably trained staff) to provide images appropriate to base clinical decision-making.
- Imaging should encompass multiple sequences (T2, DWI, ADC and DCE with calculated b-values of 1400) according to standards well established worldwide, resulting in a multi-parametric MRI study.
- Reporting should be undertaken by radiologists with appropriate training in reporting prostate MRI. Dual reporting and clinical correlation are known to improve reporting outcomes and are encouraged.
- Reports should utilise a validated and widely accepted format – PI-RADs v2 is the most widely accepted standard worldwide.
Prostate biopsy
Prostate biopsy is the definitive diagnostic tool for prostate cancer.
The biopsy pathology report includes information on the tumour grade and stage. Tumour grade relates to tumour aggressiveness and is reported using either the Gleason score or the Grade Group system, while tumour stage relates to whether the tumour is localised or whether it has spread throughout the body (summarised in the PCFA guidance booklet “Prostate cancer – a guide for newly diagnosed men[6]):
Prostate Cancer Grading
Gleason grade 1 and 2 patterns are very rarely used anymore. This means that the lowest pattern number for prostate cancer is pattern 3. There is often more than one pattern of cancer present in the biopsy. The two most common patterns of growth seen in the biopsy sample are each given a number from 1 to 5, and then these two numbers are added together to give the Gleason Score (e.g., 4 + 3 = 7).
If the first and second most common patterns in a biopsy are both pattern 3, then the Gleason Score would be 3 + 3 = 6. This is a very low-grade cancer.
If both first and second most common patterns are grade 5, the Gleason Score will be 5 + 5 = 10. These cancers are very high grade. Low grade cancers usually grow slowly and are unlikely to spread. Higher grade cancers are more aggressive, can grow more quickly and may spread to other parts of the body.
Gleason scores of 3+4 or higher are considered clinically significant, or aggressive. Gleason scores of 3+3 are considered indolent cancers that are unlikely to prove clinically significant.
Decision to proceed to prostate biopsy
The decision to proceed to prostate biopsy is made by the urologist with informed consent from the patient. The urologist will always make the decision based on their clinical judgement and experience. Factors that the urologist will consider when recommending a biopsy include the patient’s:
- overall health, co-morbidities, and blood tests,
- current medications (including blood thinning medication)
- ability to undergo anaesthesia,
- age and life expectancy,
- PSA value and trend in PSA results (stable, rising or falling),
- % free PSA result,
- most recent mpMRI result and prior mpMRI results,
- prior history of prostate cancer,
- prior prostate biopsy results,
- family history of prostate cancer,
- ethnic background,
- genetic background (e.g., BRCA2 gene mutation),
- response to (i.e. psychological impact of) a prostate cancer diagnosis, and
- their partner’s preferences (where relevant).
Shortcomings of current testing regimes
There are several shortcomings in the current testing regimes, including:
PSA screening
- Elevated PSA on its own is not a reliable indicator of prostate cancer, with only an estimated 18% of patients in the PSA 4-10ng/ml range having a positive biopsy[2].
- Elevated PSA can be caused by conditions other than prostate cancer, including prostatitis, enlargement of the prostate, bike riding and sexual activity,
- Elevated PSA cannot differentiate between patients without cancer, nor patients with non-clinically significant or clinically significant prostate cancer, and
- PSA results can vary according to the clinical analyser used, often leading to repeat testing.
mpMRI
- Patients with PI-RADs scores of 1-2 do not typically proceed to prostate biopsy, despite up to 18% of these patients having clinically significant cancers[4],
- There is no consensus on which PI-RADs 3 patients require biopsy. These patients represent a large subgroup and yet have low rates of clinically significant cancers,
- The interpretation of MRI results is subject to significant inter-operator variability,
- MRI is contraindicated for patients with contrast allergy, metal implants (pacemakers, artificial joints) or who suffer from claustrophobia,
- High quality MRI is not always available, and
- MRI is time-consuming and inconvenient to the patient.
Prostate biopsy
- Under current biopsy practice ∼40% of patients who undergo biopsy have clinically significant cancer, while 20% have non-clinically significant disease and 40% have no prostate cancer[7].
- 20% of patients are diagnosed with non-clinically significant prostate cancer, which can impact quality of life,
- Variability in prostate biopsy technique can result in missed clinically significant cancers.
MiCheck® Prostate as an additional test to aid the clinical decision to proceed to biopsy
Intended Purpose
MiCheck® Prostate is for male subjects being considered by physicians for a prostate biopsy. It is intended to supplement and not replace the significance of other clinical factors or test results which collectively assist the clinical judgement and experience of the urologist in the decision about whether to proceed to prostate biopsy. Prostate biopsy is required for the diagnosis of cancer.
MiCheck® Prostate Australia
In Australia, MiCheck® Prostate will be offered via Sonic Douglass Hanly Moir initially with plans to expand to other Australia Sonic network laboratories.
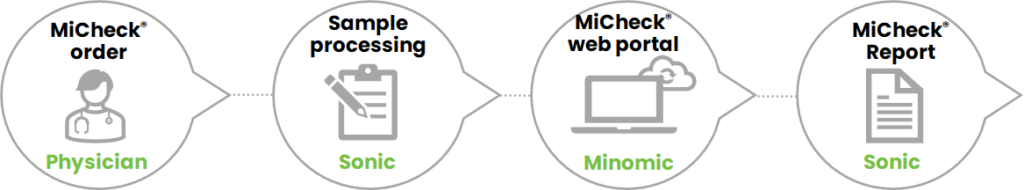
How the test will be used by the urologist in Australia
- The urologist orders the MiCheck® Prostate test using a pre-printed Douglass Hanley Moir order form containing the patient’s date of birth and PV (if available).
- The patient goes to have a blood draw at one of the Douglass Hanly Moir collection centres.
- The sample is transported to the Douglass Hanly Moir central pathology laboratory.
- The sample is tested for total PSA, free PSA and HE4.
- The results of these three markers, age and PV (if available) are reviewed and released by Douglass Hanly Moir central pathology laboratory and then uploaded to the secure and confidential MiCheck® Prostate web portal. The web portal does not require identifiable patient information.
- The web portal calculates the MiCheck® Prostate Percentage Risk Score and reports it back to Douglass Hanly Moir for incorporation into a patient report which is sent to the ordering urologist.
Advantages of adding PV/Age as clinical data to the MiCheck® Prostate algorithm
The two clinical factors (PV and age) chosen for the MiCheck® Prostate algorithm were selected as they are routinely collected during assessment of the patient by the urologist. Both these factors improve the MiCheck® Prostate test performance compared to using the blood tests alone.
In contrast to these two factors, other clinical variables such as DRE status, family history, ethnic background and genetic background were assessed for clinical performance and ease of use. None of these factors added significantly to the algorithm performance compared to age and PV. Furthermore, they are either harder to quantitate or are less frequently collected and are, therefore, less reliable to use as inputs into the algorithm.
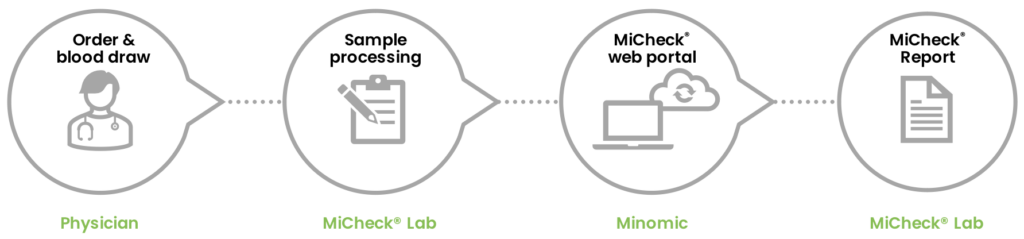
MiCheck® Prostate USA
In the United States, MiCheck® Prostate is currently offered as a Laboratory-Developed-Test (LDT) via Minomic Inc.’s CLIA laboratory at Gaithersburg (MD, USA).
Why clinical judgement overrides ANY test results
Diagnostic test results do not mandate a particular clinical course of action. The results of the MiCheck® Prostate test provide information to the urologist about the patient status and the urologist then determines the appropriate clinical course (often in consultation with the patient).
In general, the urologist will have a large amount of information available prior to recommending a prostate biopsy. Each urologist will make their own decision based on their experience and clinical judgement, and there may be differences between urologists in the recommended course of action. The final decision to recommend a biopsy is always determined by the individual urologist using their own clinical judgement based on the test results available.
[1]Carlsson, Sigrid V. “Screening and Prevention of Prostate Cancer 2021 (Part 1): Evidence for PSA Screening” May 2021. Accessed Sep 2021. https://grandroundsinurology.com/screening-and-prevention-of-prostate-cancer-2021-part-1-evidence-for-psa-screening/.
[2]NCCN Guidelines Version 2.2021 Prostate Cancer Early Detection.
[3]Kasivisvanathan et al 2018, PRECISION Study Group Collaborators. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378(19):1767 DOI: 10.1056/NEJMoa1801993.
[4]Doan et al. Identifying prostate cancer in men with non-suspicious multi-parametric magnetic resonance imaging of the prostate. ANZ J Surg 2021 Apr;91(4):578-583. doi: 10.1111/ans.16583. Epub 2021 Jan 21.
[5]Sonn et al, 2019 Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. Eur Urol Focus. 2019;5(4):592-599 doi: 10.1016/j.euf.2017.11.010. Epub 2017 Dec 7.
[6]PCFA Prostate cancer: a guide for newly diagnosed men.
[7] Shore et al, 2022. A comparison of prostate health index, total PSA, %free PSA, and proPSA in a contemporary US population—The MiCheck-01 prospective trial. Urologic Oncology: Seminars and Original Investigations, 38(8), Aug 2020, p683.e1-683.e10
https://doi.org/10.1016/j.urolonc.2020.03.011.
Get In Touch
More Info
Would you like to talk with Minomic about:
- Partnering with us to take our products to your markets?
- Media or upcoming events?
- Some feedback that you have?
- Minomic Internship Program
Address
Level 1, 3 Innovation Road, Macquarie Park NSW 2113, Australia
Australian Business Number
14 124 455 081
